Hydrometallurgy - our main subject
Hydrometallurgy - from ancient greek hydro(water), + metallurgy(the science of metals) - is the science of the behaviour of metals in aqueous solutions.
For the recovery of metals from metal containing waste, hydrometallurgy is the main treatment.
Three operations have to be considered:
-
Dissolution of the metal content in the waste (leaching) in acid or alkali, sometimes at elevated pressure and temperature. Often, a pre-treatment operation is included, like milling, grinding etc. An operation of great interest today is bio-leaching with the help of bacteria.
-
Separation of the metals - separation of different metals into pure and concentrated solutions. Operations in question are solvent extraction, ion exchange, cementation, reversed osmosis and liquid membrane technique.
-
Generation of products - the manufacture of pure metal products by electro refining, electrowinning or production of pure metal salts by evaporation, precipitation or crystallization. As indicated, there are many alternatives to carry out a complete metal recovery process, and to develop such a process you have to combine different operation steps in a unique way. The MEAB Group has documented experience and access to complete laboratory facilities for the development of hydrometallurgical processes. Our aim is to treat metal containing, solid or liquid waste in such an effective way that metals are removed from the waste and that products are produced for re-use and, finally, to leave acceptable rests for depositing in the environment.
Projects
Solvent Extraction - our speciality
Solvent extraction is a selective separation procedure for isolating and concentrating a valuable substance from an aqueous solution with the aid of an organic solution. For the production of copper, the solvent extraction technique has been accepted completely. It has been estimated that more than half of the world's copper will be produced by extraction methods according to the following procedure:
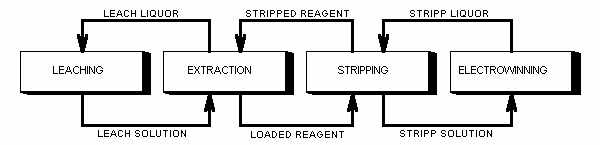
The oxidic copper ore is leached with dilute sulphuric acid. A leaching solution is prepared containing 1-6 g/1 copper together with other metals. The copper in the leaching solution is selectively extracted into a kerosene solution containing a copper selective reagent. The copper is stripped from the organic solution to a new aqueous solution so that a copper concentration of 30-35 g/1 is achieved. Pure copper is obtained from this solution by electrowinning.
Major subjects studied references
references
Keywords: Extraction, Solvent, Electrowinning, Hydrometallurgy, Leaching, Liquid-Liquid, Metal, Recovery, Centrifuge, Extractor, Mixer-Settler, EW-cell, AKUFVE, CENTREK, MSU, Countercurrent, Coextraction, Reextraction, Scrubbing, Scrub, Stripping, Mixer, Impeller, Stirrer, Mixing, Mixture, Settling, Gravity, separation, Picket, Fence, Jack-leg, Weir, Aqueous, phase, Continuous, Diluent, Dispersed, Entrainment, Extractant, Interphase, Boundary, Liquor, Outflow, Organic, Raffinate, Solubility, Solute, Solution, Inflow, Solvent, Third, phase-formation, Crud, Loading, Surface, Mass, Transfer, Degradation, Distribution, Coefficient, Constant, Partition, Factor, Ratio, Niclas, Jonas, Hans, Reinhardt, MEAB, Metallextraktion, Sweden, MX-Processer, rare, earth, minerals, elements, industrial, Research, laboratory, equipment, European,