Hydrometallurgy - from ancient greek hydro(water), + metallurgy(the science of metals) - is the science of the behaviour of metals in aqueous solutions.
For the recovery of metals from metal containing waste, hydrometallurgy is the main treatment.
Three operations have to be considered:

Solvent extraction is a selective separation procedure for isolating and concentrating a valuable substance from an aqueous solution with the aid of an organic solution. For the production of copper, the solvent extraction technique has been accepted completely. It has been estimated that more than half of the world's copper will be produced by extraction methods according to the following procedure:
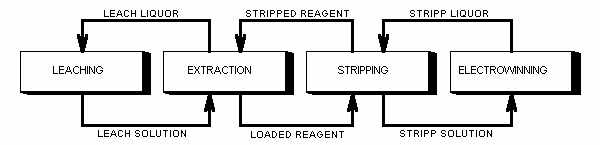
The oxidic copper ore is leached with dilute sulphuric acid. A leaching solution is prepared containing 1-6 g/1 copper together with other metals. The copper in the leaching solution is selectively extracted into a kerosene solution containing a copper selective reagent. The copper is stripped from the organic solution to a new aqueous solution so that a copper concentration of 30-35 g/1 is achieved. Pure copper is obtained from this solution by electrowinning.
 references
references
